A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between the entities in both the reactants and the products and an arrow that points towards the products and shows the direction of the reaction. In order to measure the heat of a reaction the reaction must be isolated so that no heat is lost to the environment.
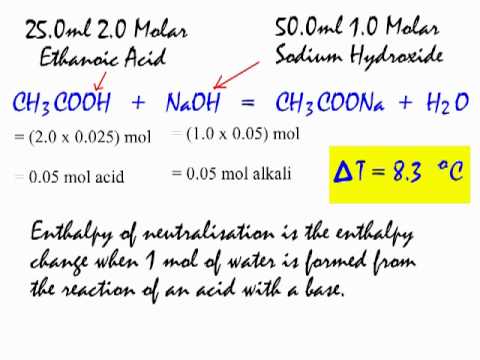
Calorimetry Determing An Enthalpy Of Neutralisation From Www Flashscience Co Uk Youtube
Given either the initial and final temperature measurements of a solution or the sign of the H rxn identify if a reaction is endothermic or exothermic.

. Yield actual yieldtheoretical yield 100 So lets say you want to do an experiment in the lab. Because calorimetry is used to measure the heat of a reaction it is a crucial part of thermodynamics. Enthalpy Change of Hydration.
A solution was made by dissolving 125 g of Na3PO4 in 12500 mL of water. It is the change in enthalpy when one mole of gaseous ions is dissolved in water to form an infinitely dilute solution. Enthalpy Change of Neutralization.
Hesss law - Hesss law states that the total energy or enthalpy change for a chemical reaction is the same whatever route is taken. The volume of the resulting solution was 128 mL. You want to measure how much water is produced when 120 g of glucose C_6H_12O_6 is burned with enough oxygen.
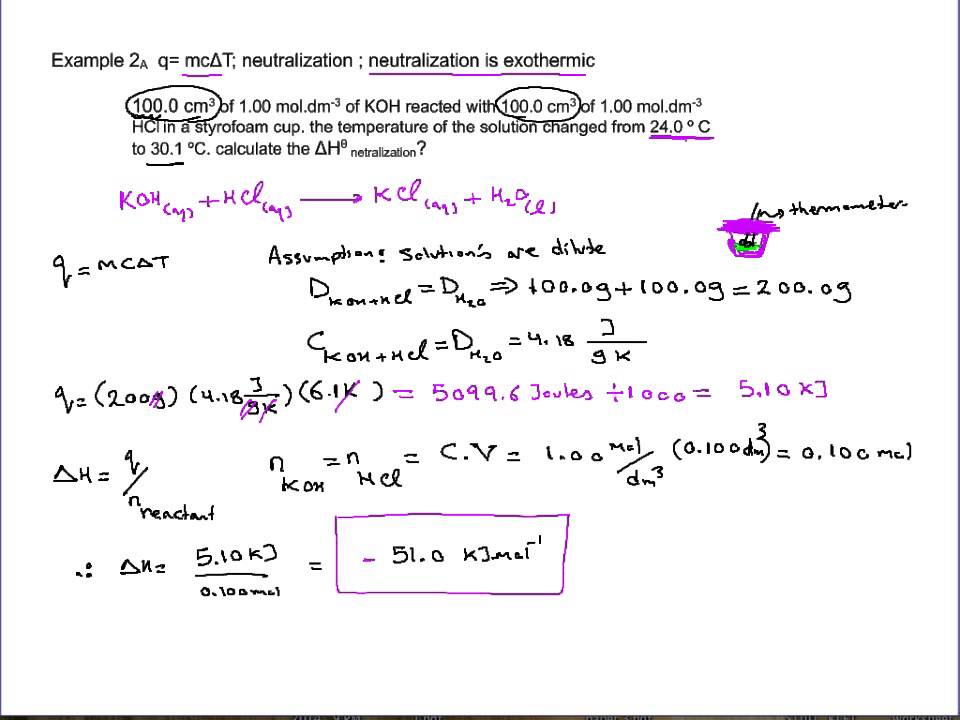
Calculate the number of moles of base you add to determine the molar heat of neutralization expressed using the equationexample suppose you add 25 mL of 10 M NaOH to your HCl to produce a heat of neutralization of 44778 Joules. In vitro studies in human cell lines and in vivo studies in a hamster model show that the SARS-CoV-2 Omicron variant is less pathogenic than both the Delta variant and an ancestral strain of SARS. Correspondingly in the live virus neutralization tests the anti-Delta neutralizing GMTs were improved by 28-fold and 64-fold respectively in.
Both the Beta and Delta variants are associated with decreased neutralization by some antireceptor-binding domain and antiN-terminal domain monoclonal antibodies although both Beta and Delta remain responsive to at least one antireceptor-binding domain 8 9 21. Learn about the anode and cathode in an electrochemical cell. Dissolution of calcium h ydroxide at each temperature is calculated from the for mula ΔG 0 - RT ln K c.
ΔG 0 values at two temperatures permit the calculation of ΔH 0 and ΔS 0. Given the change in enthalpy for a reaction the amounts of reactants and a balanced chemical equation calculate the heat exchanged for a reaction. Understand the reactions taking place on the anode and cathode of a cell.
Click to see full answer Likewise people ask how do you calculate the enthalpy of neutralization of HCl and NaOH. This is achieved by use of a calorimeter which insulates the reaction to better contain heat. Hesss law allows the combination of experimentally measured enthalpy changes to calculate the desired enthalpy change.
H sol -80 KJmol. It is always negative and is denoted by H hyd. Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100.
Na s H 2 O aq NaOH aq 12H 2 g H hyd -406 kJmol.

Enthalpy Of Neutralization Youtube

Heat Of Neutralization Part 1 Thermochemistry Youtube

Calorimetry Determing An Enthalpy Of Neutralisation From Www Flashscience Co Uk Youtube
0 Comments